The ‘281 Patent
Ranbaxy in its second action challenged the ‘281 patent validity, particularly claim 1 covering hemicalcium salt of atorvastatin on the grounds of:
(1) anticipation by the WO Application, and
(2) obviousness in the light of the Application.
Here, yet again, Judge Pumfrey followed a much careful and meticulous approach while dealing with both the anticipation and obviousness issues.
Anticipation Issue
Before deciding anticipation issue, the court laid down the principles of anticipation and went on addressing the WO Application in great detail. The court acknowledged that the WO Application is directed for ways of making particularly, Formula (I) –
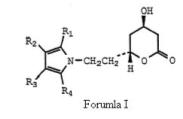 which is same of formula (I) of the ‘633 patent save that group X is explicitly ethyl, (-CH2CH2-) and more particularly directed to Formula Ia.
which is same of formula (I) of the ‘633 patent save that group X is explicitly ethyl, (-CH2CH2-) and more particularly directed to Formula Ia.
 which is same of formula (I) of the ‘633 patent save that group X is explicitly ethyl, (-CH2CH2-) and more particularly directed to Formula Ia.
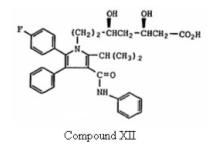
which is same of formula (I) of the ‘633 patent save that group X is explicitly ethyl, (-CH2CH2-) and more particularly directed to Formula Ia. The court further went on addressing the WO Application with particular emphasis on(1) Compound XII –
which is the ring-opened dihydroxy acid form of Formula Ia, and
(2) a statement from the WO Application stating that “the preferred isomer of this invention is the 4R, 6R-isomer of the compounds of Formula I, Ia and XII.”
The court also addressed a paragraph from the WO Application quoting –
‘In the ring-opened dihydroxy acid form, compounds of the present invention react to form salts with pharmaceutically acceptable metal and amine cations formed from organic and inorganic bases. The term "pharmaceutically acceptable metal salt" contemplates salts formed with the sodium, potassium, calcium, magnesium, aluminum, iron and zinc ions.'”
With this, the court followed that the material claimed in claim 1 is an expressly specified salt (calcium) of the preferred isomer of one of three materials explicitly specified. The court after carefully addressing the WO Application disclosure and the ‘281 patent, concluded that the final structure formula of the ‘281 patent and compound XII of the WO Application are identical, save that the ‘281 patent refers calcium salt whereas the WO Application refers the acid form. The court further concluded that the WO Application gives specific directions to make the three preferred enantiomers, and one of which falls within the claim 1 of the ‘281 patent, and thereby making a clear case of anticipation of claim 1 of the ‘281 patent.
Obviousness
While dealing obviousness issue, the dispute arises as to the right approach to obviousness in this case. Warner-Lambert took as their exemplar the decision of the Technical Board of Appeals of the European Patent Office on the present patent. On a problem-solution approach, the EPO Board of Appeal found the selection of the calcium salt and the particular enantiomer to be inventive. Judge Pumfrey found otherwise, expressing preference for the historical English approach in Windsurfing v. Tabur Marine [1985] RPC 59 (CA)) (the Windsurfer analysis) over the EPO’s problem-solution approach.
An EPO Board of Appeal in this case had been persuaded, during prosecution, to reverse an obviousness rejection on the basis that the selection of calcium from among previously described pharmaceutical metal salts (potassium, sodium, calcium, iron, aluminum, magnesium and zinc), together with selection of one of the two enantiomers from the racemic mixture, formed an inventive selection because this combination was said to improve the handling properties of the solid salt (particularly its hygroscopicity and solubility).
However, according to Judge Pumfrey, first there was no inventiveness in resolving the racemate into its enantiomers and selecting a particular enantiomer. On the evidence, there was no particular difficulty in performing resolution in this particular case.
Second, there was no inventiveness in selecting a particular salt, and least of all the calcium salt, from among the seven possibilities already disclosed. Once a pharmaceutically active component is isolated, conducting a salt screen is a standard procedure to identify the best salt for administration. Potassium, sodium and calcium salts are the most commonly used. Judge said, “We find ourselves in the strange position that if the sodium salt had been satisfactory, there would have been no invention in going for the calcium instead, but since it was not, there is an invention.”
The judge was critical of permitting the patentee to reformulate the problem on the basis of an advantage discovered after the priority date, or one known before the priority date to the patentee, but not disclosed by him in the patent application. In his own words, Judge Pumfrey stated “How can one solve an objective problem that one did not know existed?”
In his judgment, Judge Pumfrey refused to grant the declaration of non-infringement sought by Ranbaxy in respect of the ‘633 patent and invalidated the ‘281 patent for anticipation and obviousness.
Battle I Round II: U.K. Court of Appeal
Ranbaxy later appealed to United Kingdom’s Court of Appeal against the ruling of the U.K. High Court judgment regarding declaration of non-infringement in respect of the ‘633 patent. However, Court of Appeal affirmed the High Court ruling that Ranbaxy’s proposed generic product of Atorvastatin Calcium would infringe the ‘633 patent. The appeals court also affirmed High Court ruling regarding invalidity of the ‘281 patent.
To be continued...
Related Posts---
Who Took Away The Better Half? Lipitor Patent Battle
Who Took Away The Better Half? Lipitor Patent Battle II

My name is Giulia White and i would like to show you my personal experience with Lipitor.
ReplyDeleteI have taken for 9 years. I am 60 years old. I took 20 mg for 9 years and I told numerous physicians about my pain and stiffness and was told that I had arthritis and to keep taking it. I left it at home by accident when we went on vacation and within 3 days, the pain in my legs began to go away. After 2 weeks I knew it was a very dangerous medication. I went to my new physician and he wanted me to try Pravachol. Afer 4 days on it, I was in a fog and thought I had the flu. I have been off it for just 36 hours and feel better. I am an RN and should have known that I was experiencing side effects with Lipitor, but you listen to your Doctor because you trust him. I now tell my patients to trust what their bodies are telling them. Statins can't be good for anyone but the drug companies!!!!!!!!!! They keep lowering the recommended levels so that almost everyone is considered to have "high" cholesterol. If someone is 30 and on this for 30 or 40 years there is not telling what the long term effects will be.
I have experienced some of these side effects-
Joint and Muscle Pain / Stiffness.
I hope this information will be useful to others,
Giulia White
Lipitor Prescription Information