Beyond Para IV Litigation!
By this time, you would probably be well-read about Para IV battle between Ranbaxy and Pfizer, over the world’s biggest blockbuster drug – Lipitor, with latest in news that the U.S. Court of Appeals for the Federal Circuit (CAFC) has ruled partially in the favor of Pfizer and partially favor of Ranbaxy, and thereby yet again repeating last year’s the U.K. High Court split judgment concerning infringement dispute over Pfizer’s corresponding European patents protecting atorvastatin (EP247633 or the ‘633 patent) and atorvastatin calcium (EP409281 or the ‘281 patent), famously referred as genus and species patent. But this time, the concluding parameters seems to be different with Ranbaxy finally getting a taste of euphoric success by getting a rightful chance to make an early move into US Lipitor market, around 15 months prior than the expected June 2011 deadline while Pfizer likely to approach the USPTO to rectify its technical defect in writing dependent claim of the U.S. Patent No. 5,273,995 (the ‘995 patent) which eventually cost Pfizer the critical Para IV litigation. Apart from these distinctive parameters, there are few appealing questions that still need to be encountered.
First, is there any difference of opinion in the U.K. and U.S. judgments?
Secondly, who really hits the bottom-line? Is it Ranbaxy or is it Pfizer?
Thirdly and the most important, who really took away the better half? Is it Pfizer or is it Ranbaxy?
Let’s try to figure out these intriguing questions in light of earlier decided the U.K. High Court, U.K. Court of Appeals and U.S. District Court judgments, and recently decided CAFC ruling!
Targeting Lipitor!
Ranbaxy became the first generic company to attack Pfizer’s key patents protecting the best-selling drug – Lipitor, challenging their validity in number of jurisdictions including U.S. and U.K., even though, it tasted very limited success and that too with no commercial interest as Pfizer went on defending its patents in Finland, Norway, Romania and Peru but loses Austrian patent covering atorvastatin calcium to Ranbaxy. A number of these decisions are being later appealed by Ranbaxy with higher courts in respective countries. However, Ranbaxy tasted its major victory on October 12, 2005 when the Judge Nicholas Pumfrey of the U.K. High Court invalidated the ‘281 patent but subsequently found that the Ranbaxy’s proposed atorvastatin calcium would infringe the ‘633 patent. This ruling, as such, was of no commercial value to Ranbaxy but gave Ranbaxy a much-needed moral victory for its parallel Para IV litigation in the U.S. District Court for the District of Delaware.
Battle I Round 1: The U.K. High Court
Earlier in the High Court of Justice Chancery Division Patents Court, Ranbaxy brought two actions against Warner-Lambert (subsidiary of Pfizer) seeking declaration of non-infringement in respect of the ‘633 patent and distinctively seeking revocation of the ‘281 patent. During trial Judge Pumfrey dealt with the following issues –
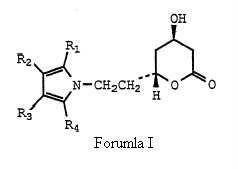
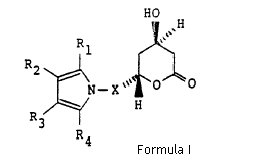
Claim construction with respect of the ‘633 patent. In specific, does the claim illustrating structural compound of Formula (I) cover the single enantiomers, or does in only cover racemic atorvastatin which according to Ranbaxy was illustrated by Formula (I) when properly construed.
Obviousness and Anticipation issues with respect to the ‘281 patent. In specific, whether the ‘281 patent is
(a) obvious over the application for the ‘633 patent (the Application), and
(b) anticipated by Warner-Lambert’s application with PCT publication number WO 89/07598 (the WO Application).
To be continued…