Friday, February 23, 2007
Centre Defended Section 3(d)
Wednesday, February 14, 2007
Topamax Patent Litigation: US Court favors Johnson & Johnson
District Judge Stanley Chesler of the US District Court for the District of New Jersey has ruled in the favor of Johnson & Johnson in an infringement suit over its U.S. Patent No. 4,513,006 (the ‘006 patent) for epilepsy drug Topamax, generically known as Topiramate and thus prohibiting Mylan to sell generic Topamax before expiry of the ‘006 patent. In October 2006, J&J also won a preliminary injunction against Mylan which got US FDA approval for its generic Topamax in September 2006. Mylan during court proceedings argued that the ‘006 patent on Topamax was invalid because its subject matter would have been “obvious” to a person having ordinary skill in the drug research. Judge Chesler, in his Feb. 02 ruling, said he found Mylan’s arguments unpersuasive. He said the generic drugmaker "has not presented even a theory of obviousness."
Saturday, February 10, 2007
Medtronic Loses Patent Lawsuit against BrainLAB
US Court of Appeals for the Federal Circuit has upheld the US District Court for the District of Colorado judgment to dismiss the patent infringement lawsuit filed by Medtronic against BrainLAB in 1998, affirming the district court ruling that BrainLAB image-guided surgery and radiotherapy products do no infringe any of the patents in suit (US Patent Nos. 4,722,056; 5,386,454; 5,389,101 and 5,603,318). In February 2006, the US District Court dismissed Medtronic’s patent infringement lawsuit, finding that BrainLAB products do not infringe any of Medtronic’s patents in suit. The district court concluded that a September 2005 jury verdict, where the jury found that BrainLAB infringed four patents held or licensed by Medtronic and awarded Medtronic US $ 51 million, was is error and not supported by the evidence.
QLT Settles Patent Dispute over Eligard
Sanofi Loses Lovenox Patent Lawsuit
Wednesday, February 07, 2007
After Repligen, Abbott Suing ImClone Systems for Erbitux

Monday, February 05, 2007
Novartis received Indian Patent for Benflumetol derivatives
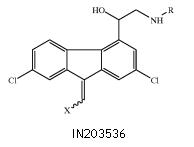
Merckle receives Indian Patent for 4-Pyridyl-and 2, 4-pyrimidinyl-substituted pyrrole derivatives

Sunday, February 04, 2007
Wyeth in Trouble over Zosyn Formulation Patent

Chennai-based Orchid Chemicals & Pharmaceuticals Ltd. along with two other generic manufactures --- Sandoz and Abraxis Pharmaceutical Products sued Wyeth for unfairly delaying the entry of generic versions of blockbuster Zosyn in the US market. All three generic manufactures filed citizen petitions with the USFDA against Wyeth, after Wyeth received the U.S. Patent No. 6,900,184 (the ‘184 patent) on its blockbuster drug Zosyn, extending its patent life to 2023. U.S. Patent No. 4,562,073 (the ‘073 patent) covering tazobactam sodium, one of the key active ingredients of Zosyn will be running out patent protection on February 09, 2007. Wyeth discontinued its original formulation for Zosyn in November 2005 and subsequently launched a new patented formulation, delaying generic entry in the US market. According to the US FDA rules, a company is allowed to withdraw a product from the market only for reasons related to safety and efficacy. In case the product is withdrawn from the market, it is subsequently removed from the approved drugs’ reference list, and therefore no pharmaceutical company can market a generic version of this same drug. The generic manufactures challenged Wyeth approach, arguing that Wyeth is trying to extend the paten life of the drug and prevent the entry of generic players in the U.S. market. Generics further added while Wyeth has added an excipient to its drug, other players should be allowed to market generic versions of the original formulation for Zosyn. Orchid, Sandoz and Abraxis pharma already filed ANDAs in order to market a generic version of Zosyn’s original formulation in the U.S. The ‘184 patent particularly covers Wyeth’s re-launched Zosyn formulation comprising cryodesiccated powder of piperacillin sodium and tazobactam sodium along with edentate disodium dehydrate (EDTA) as a particulate formation inhibitor and sodium citrate as a buffer.