Monday, May 08, 2006
Sunitinib Malate - New Drug Approved by US FDA
International Non-Proprietary Name: Sunitinib Malate
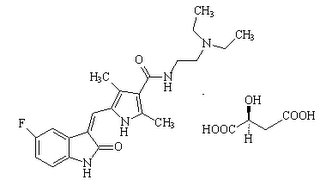
Chemical Name: N-[2-(diethylamino) ethyl]-5-[(Z)-(5-fluoro-2-oxo-1, 2-dihydro-3H-indol-3-ylidene) methyl]-2, 4-dimethyl-1H-pyyrole-3-carboxamide (2S)-2-hydroxybutanedioate
Molecular Formula: C22H27FN4O2 . C4H6O5
Chemical Structure:
CAS Number: 341031-54-7
Brand Name: Sutent
Dosage Form: Capsule (12.5mg, 25mg, 50mg)
Applicant: Pfizer
U.S. Patent No. 6,573,293
U.S. Patent Expiry: February 15, 2021
Data Exclusivity Expiry: January 26, 2011
Therapeutic Indication: Treatment of Cancer
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment