The ‘633 Patent: Determining Structural Scope
With respect to the ‘633 patent, Ranbaxy contended that its atorvastatin calcium will comprise the single optically pure enantiomer of atorvastatin calcium trihydrate salt and would not constitute infringement of any of the claims of the ‘633 patent. In particular, Ranbaxy argued regarding the scope of structural Formula (I) of claim 1 of the ‘633 patent which is reproduced below for supportive reference.
1. A compound of structural formula (I)
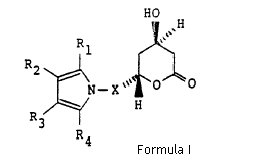 wherein X is -CH2-, -CH2CH2-, -CH2CH2CH2- or -CH2CH(CH3)-; R1 is 1-naphthyl; 2-naphthyl; cyclohexyl; norbornenyl; 2-, 3-, or 4-pyridinyl; phenyl, phenyl substituted with fluorine, chlorine, bromine, hydroxyl; trifluoromethyl; alkyl of from one to four carbon atoms, alkoxy of from one to four carbon atoms, or alkanoyloxy of from two to eight carbon atoms; either of R2 or R3 is -CONR5R6 where R5 and R6 are independently hydrogen; alkyl of from one to six carbon atoms; 2-, 3-, or 4-pyridinyl; phenyl; phenyl substituted with fluorine, chlorine, bromine, cyano, trifluoromethyl, or carboalkoxy of from three to eight carbon atoms; and the other of R2 or R3 is hydrogen; alkyl of from one to six carbon atoms; cyclopropyl; cyclobutyl, cyclopentyl, cyclohexyl; phenyl; or phenyl substituted with fluorine, chlorine, bromine, hydroxyl; trifluoromethyl; alkyl of from one to four carbon atoms, alkoxy of from one to four carbon atoms, or alkanoyloxy of from two to eight carbon atoms; R4 is alkyl of from one to six carbon atoms; cyclopropyl; cyclobutyl; cyclopentyl; cyclohexyl; or trifluoromethyl; or a hydroxy acid or pharmaceutically acceptable salts thereof, derived from the opening of the lactone ring of the compounds of structural formula I and having the formula X
wherein X is -CH2-, -CH2CH2-, -CH2CH2CH2- or -CH2CH(CH3)-; R1 is 1-naphthyl; 2-naphthyl; cyclohexyl; norbornenyl; 2-, 3-, or 4-pyridinyl; phenyl, phenyl substituted with fluorine, chlorine, bromine, hydroxyl; trifluoromethyl; alkyl of from one to four carbon atoms, alkoxy of from one to four carbon atoms, or alkanoyloxy of from two to eight carbon atoms; either of R2 or R3 is -CONR5R6 where R5 and R6 are independently hydrogen; alkyl of from one to six carbon atoms; 2-, 3-, or 4-pyridinyl; phenyl; phenyl substituted with fluorine, chlorine, bromine, cyano, trifluoromethyl, or carboalkoxy of from three to eight carbon atoms; and the other of R2 or R3 is hydrogen; alkyl of from one to six carbon atoms; cyclopropyl; cyclobutyl, cyclopentyl, cyclohexyl; phenyl; or phenyl substituted with fluorine, chlorine, bromine, hydroxyl; trifluoromethyl; alkyl of from one to four carbon atoms, alkoxy of from one to four carbon atoms, or alkanoyloxy of from two to eight carbon atoms; R4 is alkyl of from one to six carbon atoms; cyclopropyl; cyclobutyl; cyclopentyl; cyclohexyl; or trifluoromethyl; or a hydroxy acid or pharmaceutically acceptable salts thereof, derived from the opening of the lactone ring of the compounds of structural formula I and having the formula X
 wherein X is -CH2-, -CH2CH2-, -CH2CH2CH2- or -CH2CH(CH3)-; R1 is 1-naphthyl; 2-naphthyl; cyclohexyl; norbornenyl; 2-, 3-, or 4-pyridinyl; phenyl, phenyl substituted with fluorine, chlorine, bromine, hydroxyl; trifluoromethyl; alkyl of from one to four carbon atoms, alkoxy of from one to four carbon atoms, or alkanoyloxy of from two to eight carbon atoms; either of R2 or R3 is -CONR5R6 where R5 and R6 are independently hydrogen; alkyl of from one to six carbon atoms; 2-, 3-, or 4-pyridinyl; phenyl; phenyl substituted with fluorine, chlorine, bromine, cyano, trifluoromethyl, or carboalkoxy of from three to eight carbon atoms; and the other of R2 or R3 is hydrogen; alkyl of from one to six carbon atoms; cyclopropyl; cyclobutyl, cyclopentyl, cyclohexyl; phenyl; or phenyl substituted with fluorine, chlorine, bromine, hydroxyl; trifluoromethyl; alkyl of from one to four carbon atoms, alkoxy of from one to four carbon atoms, or alkanoyloxy of from two to eight carbon atoms; R4 is alkyl of from one to six carbon atoms; cyclopropyl; cyclobutyl; cyclopentyl; cyclohexyl; or trifluoromethyl; or a hydroxy acid or pharmaceutically acceptable salts thereof, derived from the opening of the lactone ring of the compounds of structural formula I and having the formula X
wherein X is -CH2-, -CH2CH2-, -CH2CH2CH2- or -CH2CH(CH3)-; R1 is 1-naphthyl; 2-naphthyl; cyclohexyl; norbornenyl; 2-, 3-, or 4-pyridinyl; phenyl, phenyl substituted with fluorine, chlorine, bromine, hydroxyl; trifluoromethyl; alkyl of from one to four carbon atoms, alkoxy of from one to four carbon atoms, or alkanoyloxy of from two to eight carbon atoms; either of R2 or R3 is -CONR5R6 where R5 and R6 are independently hydrogen; alkyl of from one to six carbon atoms; 2-, 3-, or 4-pyridinyl; phenyl; phenyl substituted with fluorine, chlorine, bromine, cyano, trifluoromethyl, or carboalkoxy of from three to eight carbon atoms; and the other of R2 or R3 is hydrogen; alkyl of from one to six carbon atoms; cyclopropyl; cyclobutyl, cyclopentyl, cyclohexyl; phenyl; or phenyl substituted with fluorine, chlorine, bromine, hydroxyl; trifluoromethyl; alkyl of from one to four carbon atoms, alkoxy of from one to four carbon atoms, or alkanoyloxy of from two to eight carbon atoms; R4 is alkyl of from one to six carbon atoms; cyclopropyl; cyclobutyl; cyclopentyl; cyclohexyl; or trifluoromethyl; or a hydroxy acid or pharmaceutically acceptable salts thereof, derived from the opening of the lactone ring of the compounds of structural formula I and having the formula X
where X, R1, R2, R3 and R4 are as defined above.
During hearing Ranbaxy further argued that although two structural formula (I) and (X) appear to show (R*, R*) compound but in context of claim construction is only limited to the racemate and does not cover the single enantiomers as it is common in organic chemistry to use the structural formula of a single enantiomer to denote the racemate. Ranbaxy further supported its contention by addressing the specification disclosure, contending that the specification makes it clear that the compound of claim 1 is concerned is the racemate, and that in its context formula (I) is being used to refer exclusively to the racemate.
However, before construing the scope of structural claim the Judge Pumfrey acknowledged that claim is couched in highly technical language and uses the device of the chemical structure formula to convey its meaning. The judge further explained that illustrated structural formula shows, in highly schematic way, how the chemical bonds are located between atoms of the molecule. The judge also admitted that apart from the myriad of different substitutions that are permitted by reference to the components X and R1-4, the questions is what possible 3-dimensional arrangements of the molecule are covered by the claim, that is, its stereochemical interpretation.
Before reaching his conclusion, the judge carefully went on to identify the skilled person to which the ‘633 patent is addressed and the relevant part of the common knowledge regarding stereochemistry and statins prevailing at the time of filing the application for the ‘633 patent. In identifying skilled person, the court acknowledged that the EP ‘633 patent is intended for those who will synthesis an active ingredient and formulate it for use in therapy as a hypolipidaemic or hypocholestrolaemic agent, and thus the EP ‘633 patent is directed towards medicinal chemists with skills in organic synthesis.
After determining the level of skilled person, the court further went on to state common general knowledge regarding the subject-matter and acknowledged that the case is concerned with stereochemistry and explained few technical terminologies used during the court hearing and trial, namely, enantiomers, diastereoisomers, and racemate.
The court also acknowledged that different enantiomers of a chiral molecule react differently with other chiral molecules and which is particular importance in natural systems since enzymes, which are proteins responsible for all the chemical reactions carried out by the cell, are chiral molecules and are present only as a single enantiomer. The court further acknowledged that within a chiral environment the two enantiomers of a racemate are totally different compounds and very often the majority of the biological activity observed for a racemate resides within a single enantiomer (citing the example of Thalidomide). The court further moves on, to state common general knowledge in relation to statins addressing that the first statins, mevinolin and compactin, were natural products that existed as single enantiomers.
The court critically analyzed and addressed the specification disclosure of the ‘633 patent, pointing out a short but significant paragraph at page 4 lines 8 to 12 quoting –
“The compounds of structural formula I above possess two asymmetric carbon centers, one at the 4-hydroxy position of the pyran-2-one ring, and the other at the 6-position of the pyran-2-one ring where the alkylpyrrole group is attached. This asymmetry gives rise to four possible isomers, two of which are the R-cis- and S-cis-isomers and the other two of which are the R-trans- and S-trans-isomers. This invention contemplates only the trans- form of the compounds of formula I above.” Further, the court also acknowledged that exemplified examples, particularly Examples 1, 3 and 4 of the ‘633 patent produce racemate form of atorvastatin.
Finally, the court pointed out that the dependent claims 3, 4 and 5 denotes compound rather than a structural formula where only claim 3 include the (±) symbol to denote the racemate. Indeed, a small but legally relevant point to consider.
Judge Pumfrey, after carefully applying applicable principles of claim construction, and meticulously identifying the skilled person to which the ‘633 patent is addressed and the common general knowledge regarding stereochemistry and statins, in his judgment ruled that “every time the skilled person go through formula I or formula X he will see it with eyes that tell him that in that racemate, there is a single enantiomer that is the effective compound, and that he can resolve the racemate using conventional techniques to extract that enantiomer” and thereby concluded that claim covers both the racemate and the individual enantiomers, and refused the declaration for non-infringement sought by Ranbaxy.
To be continued…

No comments:
Post a Comment